Abstract
Introduction: Daratumumab in combination with cyclophosphamide, bortezomib and dexamethasone (Dara/CyBorD) is the only FDA approved therapy for newly diagnosed systemic light-chain (AL) amyloidosis (N Engl J Med 2021;385:46). Belantamab mafodotin is a novel anti-BCMA immunoconjugate with humanized IgG1 anti-BCMA monoclonal antibody conjugated to a microtubule-disrupting agent, monomehtyl auristatin F (MMAF) via a non-cleavable linker (Blood 2014;123:3128). Phase I/II studies in heavily pre-treated multiple myeloma patients showed single agent clinical activity with overall response rates ranging from 30-60%, with majority of responses being durable at 13 months of follow-up. Toxicity profile included keratopathy, thrombocytopenia and anemia (Blood Cancer J 2019;9:37; Lancet Oncol 2020;21:207). Based on these results, belantamab mafadotin (BLM; Blenrep) was FDA approved for relapsed myeloma. A role for new agents such as BLM in AL has not been previously reported. Here we report outcomes of six patients who received BLM at different centers for relapsed refractory (RR) AL associated with myeloma.
Methods: In this retrospective study we identified AL patients with RR disease who received at least one dose of BLM. In a multi-institutional collaboration we collected demographic, medical history, laboratory, pathologic and treatment/response data on patients with myeloma and biopsy-proven AL who had received BLM. Laboratory assessment including evaluations for hematologic and organ response was done as per standard criteria and toxicity assessed as per CTCAE v6.0.
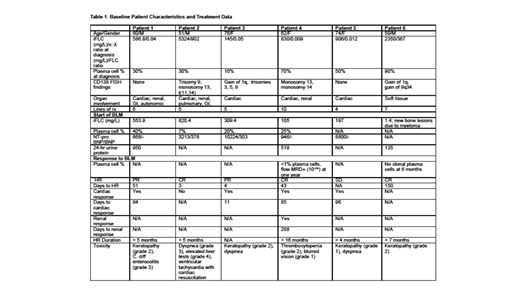
Results: We identified 6 patients, 3M/3F, from 4 centers; baseline characteristics and treatment data are provided in Table 1. Baseline median age was 61 years (range, 51-74) and median marrow plasmacytosis and iFLC were 40% (10-90) and 868mg/L (145-5324). Four patients had AL λ-type and 2 κ-type, and 5 of 6 had cardiac involvement while 3 had additional organ involvement (renal, GI, nervous system). Prior to initiating BLM the median number of lines of prior therapy was 6 (range, 5-10), including daratumumab, bortezomib and lenalidomide, and prior to initiating BLM marrow assessment showed a median plasmacytosis of 23%. BLM at 2.5 mg/kg was given as an intravenous infusion over the course of 30 minutes every three weeks after ophthalmologic exam clearance until discontinuation for progression or toxicity. At a median follow-up of 4.5 months, 5 patients (83%) achieved hematological responses (HR) with 3 (50%) achieving complete hematological responses (CR) by standard criteria (J Clin Oncol 2012;30:4541). Time to HR ranged from 3 to 150 days. Cardiac response was seen in all but 1 patient, with time to response ranging from 11 to 96 days. One patient had a renal response; response assessment is not yet available for 2 other patients with renal involvement. The most common toxicity was keratopathy (grade 1-2).
BLM was held after the first dose in one patient who had been heavily pre-treated and had extensive cardiac and pulmonary AL and multiple sites of FDG-avid progressive myeloma bone disease. Two days after administration of the first dose of BLM, this 51-year-old man was admitted to hospital for dyspnea, developed atrial fibrillation and ventricular tachycardia, and briefly required cardiac resuscitation without intubation with return of spontaneous circulation after 6 minutes. This patient achieved a CR after one dose of BLM that has been stable for over 5 months with marked clinical improvement. A 62 year-old woman with cardiac and renal AL has achieved a CR durable for over 16 months with cardiac and renal responses.
Conclusions: In this group of 6 patients with RR AL with myeloma, HR and cardiac response rates were impressive at 83% and 80%, respectively. One patient who had 24-hour urine protein evaluation also achieved a renal response. Time to response was rapid with 2 patients achieving HR within a week of starting treatment, and the rest within five months. Additionally, 3 of 6 patients achieved CR, 1 had no clonal plasma cells in the marrow and another clonal disease detectable only by MRD. In this retrospective multi-institutional cohort BLM resulted in rapid reduction of iFLC and induced critical organ responses. These data provide preliminary evidence for the clinical activity of BLM in RR AL. Results of the on-going phase 2 clinical trial in the European Myeloma Network (EMN27; NCT04617925) are awaited with great interest.
Sborov: Sanofi: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy; SkylineDx: Consultancy. Comenzo: Karyopharm: Research Funding; Prothena Biosciences: Consultancy, Research Funding; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Caelum: Consultancy, Research Funding; Janssen: Patents & Royalties: WO2016187546A1, Research Funding. Kansagra: Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Cota Health: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Alynylam: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal